Zahedan University of Medical Sciences launches Covid-19 vaccination campaign
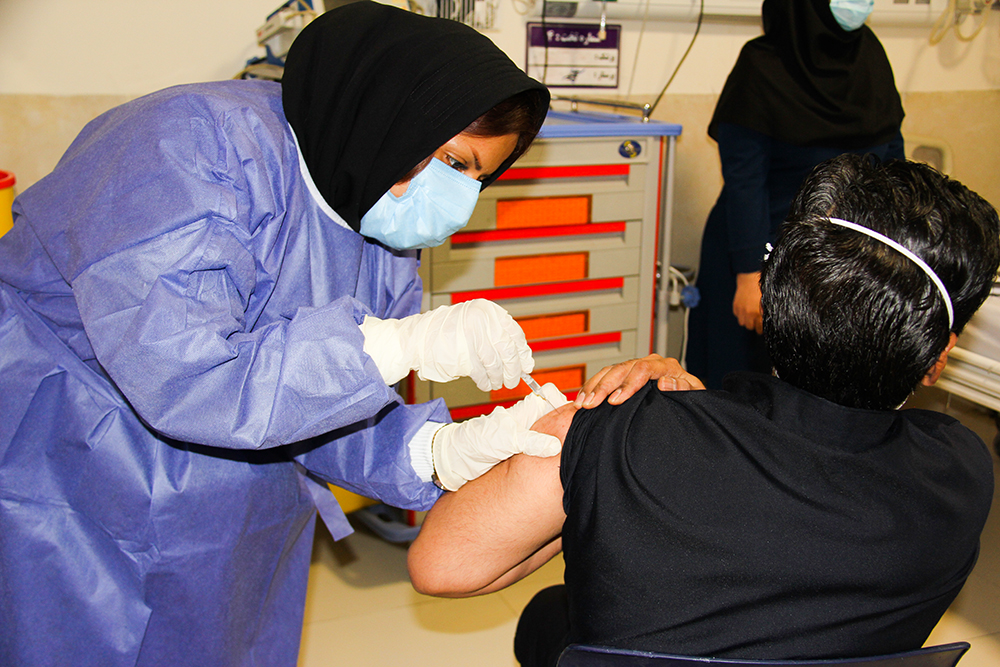
Iran began its Covid-19 vaccination campaign on Tuesday 11 February 2021, starting with front-line healthcare and medical workers as the country attempts to contain the coronavirus disease epidemic in the country.
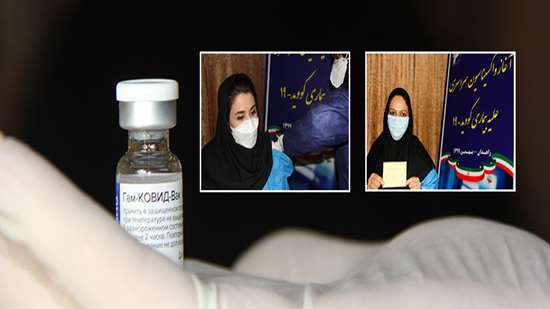
Report from: Zahedan University of Medical Sciences, Vice Chancellery for Health
Published on: 13 February 2021
Iran began its Covid-19 vaccination campaign on Tuesday 11 February 2021, starting with front-line healthcare and medical workers as the country attempts to contain the coronavirus disease epidemic in the country.
The son of Iran"s Minister of Health H.E. Dr. Saeed Namaki, Mr. Parsa Namaki, who is not a health professional, became the first recipient of the vaccine.
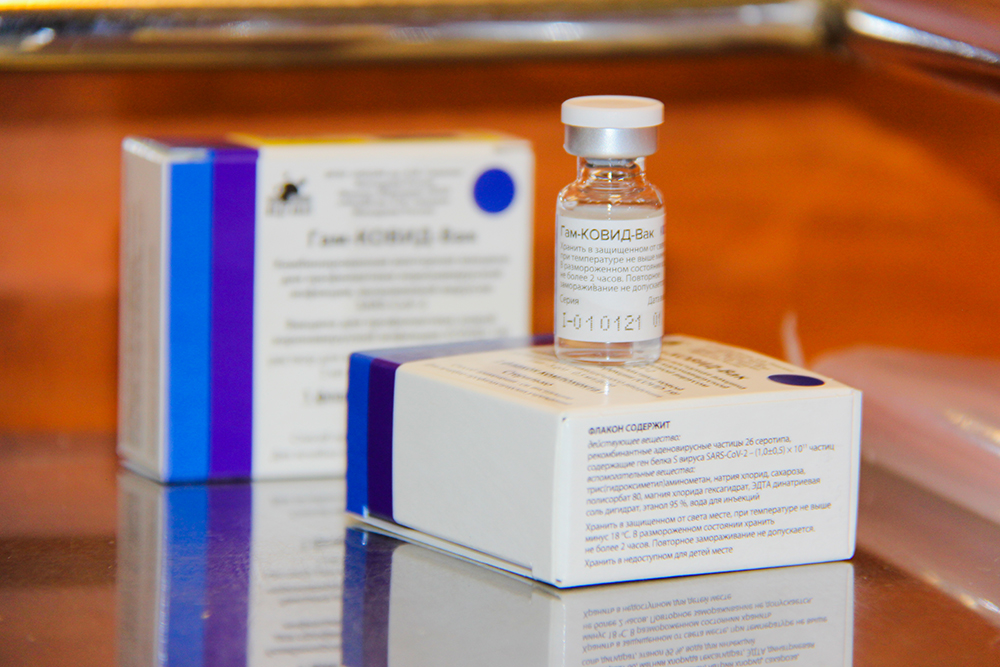
According to the Iran"s Ministry of Health authorities, Covid 19 vaccination campaign started with Russia"s Sputnik V vaccine. The Islamic republic has bought two million doses of Sputnik V produced by Gamaleya Research Center, Russia.
The Gamaleya National Center of Epidemiology and Microbiology is one of the world"s leading research institutions which as founded in 1891. The center runs one of the unique "virus libraries" in the world and has its own vaccine production facility. Gamaleya Research Center has recently received an international patent for Ebola vaccine using adenovirus vector.
The report of the interim results from a phase 3 trial of the Sputnik V COVID-19 vaccine published in The Lancet highlighted a consistent strong protective effect across all participant age groups.
The Russian Sputnik V vaccive efficacy rate is almost equal to vaccines developed by Moderna Inc. and Pfizer Inc. and its German partner BioNTech SE, which are around 95% effective, and outshines the vaccine produced by the British-Swedish multinational pharmaceutical company AstraZeneca PLC, which has published efficacy rates at between 62% to 90% in late clinical trials, with most trial results in the lower end of that range.
Sputnik V vaccine efficacy, based on the numbers of confirmed COVID-19 cases from 21 days after the first dose of vaccine, is reported as 91.6% (95% CI 85.6-95.2), and the suggested lessening of disease severity after one dose is particularly encouraging for current dose-sparing strategies. No serious adverse events considered related to the vaccine have been reported.
Please see the following link for the latest updates on covid 19 vaccines:
H.E. Health Minister Dr. Saeed Namaki said last week that Iran would also receive 4.2 million doses of the vaccine developed by Anglo-Swedish firm AstraZeneca and Oxford University, purchased via the international vaccine mechanism Covax.
Iran"s Pasteur Institute is planning to produce a vaccine named Soberana 2 in collaboration with Cuba"s Finlay Institute.
Iran is also testing its first coronavirus vaccine, called Coviran Barekat, which will be rolled out in early April.
The country also launched the first phase in the human trial of its second COVID-19 vaccine developed by the Razi Vaccine and Serum Research Institute dubbed Razi COV-Pars.
Tehran, Moscow agree on joint production of Sputnik V in Iran
It is worthwhile mentioning that the virus needs the spike protein in order to be able to infect cells. The spike protein binds to the ACE2 receptor of human cells. The virus then fuses with the cell membrane and releases its genetic material into the cell interior. However, the exposed location of the spike protein on the viral surface also makes it an important target for the immune system. It is therefore the focus of the development of vaccines and antiviral therapeutics.
Copyright © 2021 Zahedan University of Medical Sciences. All rights reserved. Date updated: 13/02/2021
Should you have any queries please do not hesitate to contact us on: zu.healthdeputy@gmail.com




















comment